High Myopia Clinic In Tokyo Medical and Dental University
What is High Myopia Clinic?
High Myopia Clinic in Tokyo Medical and Dental University was established by honorary Prof. Takashi Tokoro in 1974 as the world only clinic specific to pathologic myopia. Now our High Myopia Clinic has the longest history for the management of highly myopic patients in the world and the registered patients exceed > 3,ooo. Our university is located at the center of Tokyo with good traffic access from Haneda or Narita International Airport. We believe that we always provide the best possible care for highly myopic patients based on our consistent world-leading study results.

|
Founder and advisory chief |
Honorary Prof. Takashi Tokoro | Members |
Takeshi Yoshida Noriaki Shimada Ariko Miyazawa Muka Moriyama Kengo Hayashi Hideaki Tobita Shin-ichi Sueyoshi Natsuko Nagaoka Natsuko Inui Kaori Kasahara Yuichiro Tanaka Tae Yokoi Kosei Shinohara Yuichiro Kaneko |
|---|---|---|---|
| Chief |
Kyoko Ohno-Matsui

|
What is Pathologic Myopia?
How important as a cause of vision loss?
Pathologic myopia is also called as high myopia or degenerative myopia, and all of them mean the same pathology.
The excessive increase of the length of eye in anterior-posterior direction (axial length) is the essential feature of pathologic myopia.
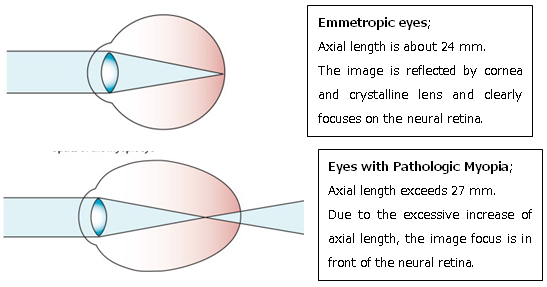
In addition to optical defocus due to excessive increase of axial length, the posterior segment of the eye is stretched and deformed, as shown by 3D MRI.
Such deformity of the posterior segments of the eye stretches, thins, and damages the neural retina and the optic nerve, which eventually develops un-corrected progressive visual loss.
Actually, pathologic myopia is a leading cause of visual impairment, and is the fourth to ninth most frequent cause of blindness worldwide. In Asian countries, pathologic myopia is the most frequent cause of visual impairment; the Tajimi Study in Japan showed that myopic macular degeneration is the leading cause of unilateral or bilateral blindness, whereas in China, it is the second most common cause of low vision/blindness in participants who were >40 years of age according to the World Health Organization.
Visual impairment in eyes with pathologic myopia is mainly due to the development of different types of myopic maculopathies. In highly myopic eyes, the axial elongation of the eye and the development of a posterior staphyloma result in a thinning of the retina and choroid, which then lead to the development of different types of myopic maculopathy. The impact of myopic maculopathy on visual impairment is important because the maculopathy is often bilateral, irreversible, and frequently affects individuals during their most productive years.8
Why Pathologic Myopia causes un-corrected visual impairment?
Eye globe contains the central nerve tissue inside the scleral shell. Thus, a marked elongation and deformity of the posterior sclera causes thinning and damage of the neural retina as well as the optic nerve, both of which are critically important for vision. These lesions are explained in detail in the following chapters.
Representative Myopic fundus lesions
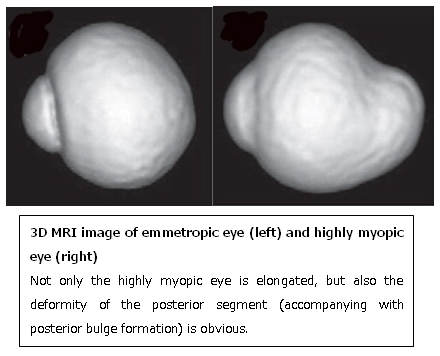
Posterior staphyloma
Although posterior staphyloma itself does not directly causes vision loss, other macular lesions can develop more frequently in highly myopic eyes with staphyloma than those without staphyloma. Thus, the detection of staphyloma is important for clinical management of highly myopic patients.
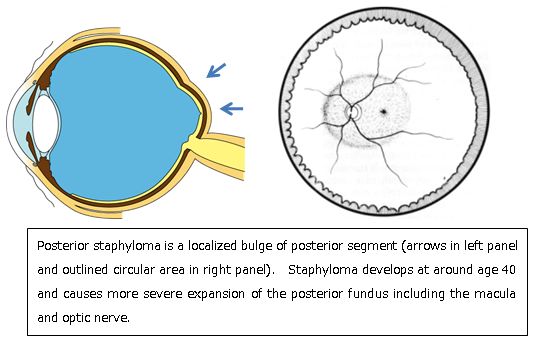
Myopic choroidal neovascularization (myopic CNV)
Pathologic myopia is the leading cause of macular CNV in the patients younger than 50 y/o. CNV develops in 10% of highly myopic eyes, and eventually affect bilaterally in 1/3 of the cases. When myopic CNV develops, the patients complain sudden loss of central vision or distorted vision. Different from CNV due to age-related macular degeneration, the CNV tends to be small and does not accompany extensive hemorrhages (in often cases). To diagnose and determine the activity of CNV, fundus examination alone might not be sufficient. A combination of optical coherence tomography and fluorescein fundus angiography is highly recommended.

Myopic chorioretinal atrophy
Due to a marked stretching and thinning of retina-choroid, various types of chorioretinal atrophy develop in the macular area. Usually, chorioretinal atrophy does not involve the central fovea. Thus, the main symptom is a gradual enlargement of visual field defects. When patients complain central vision loss although only chorioretinal atrophy is observed in the fundus examination, the ophthalmologists should suspect some other lesions than chorioretinal atrophy and thorough examinations are highly recommended. Unfortunately, there are no useful therapies to prevent or treat chorioretinal atrophy at present.
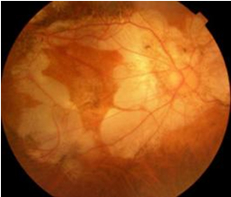
Myopic traction maculopathy (MTM)
MTM was first reported by Prof. Shoji Kishi in Gunma University as predisposing lesion for myopic macular hole retinal detachment. Due to an extensive stretch of neural retina in the posterior fundus, the retina eventually splits in the outer retinal layer (macular retinoschisis).
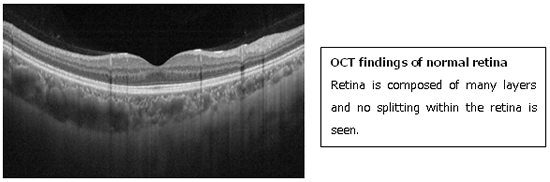
The diagnosis of MTM is made by optical coherence tomography (OCT).
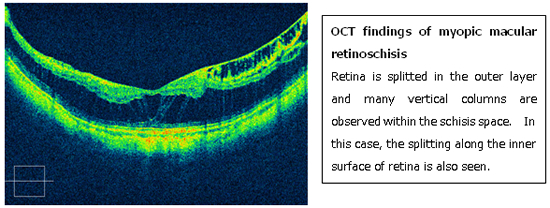
In natural course, macular retinoschisis progresses to more severe complications like full-thickness macular hole of macular retinal
Patients usually complain the central vision loss or distorted vision. However in early stages, they do not complain obvious symptoms. Thus, in highly myopic patients, the ophthalmologists should examine them by using OCT regularly even though they do not complain visual symptoms.
Surgical treatments are necessary for the cases who are considered to develop more serious complications.
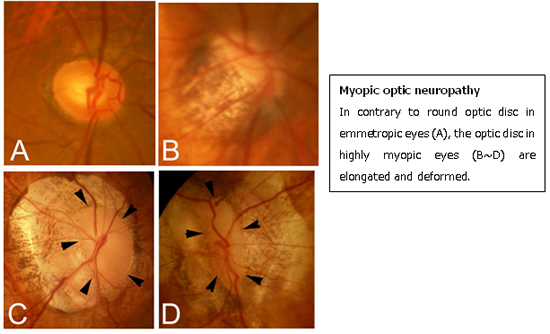
Myopic optic neuropathy
Due to a mechanical stretching and deformity of lamina cribrosa as well as peripapillary sclera, the optic nerve is also deformed. The optic nerve damage in highly myopic eyes is progressive and in some cases results in total blindness. Despite its clinical significance, the presence of optic nerve damage is often missed because the evaluation of such deformed optic disc is difficult. Regular check-up by visual field examination is recommended for highly myopic eyes.
We have recently been clarifying the pathogenesis and pathologies which could cause optic nerve damage in highly myopic eyes by using the most recent technology; swept-source OCT and 3D MRI analyses.
Updated treatment strategies against myopic fundus lesions
Anti-VEGF therapies against myopic CNV
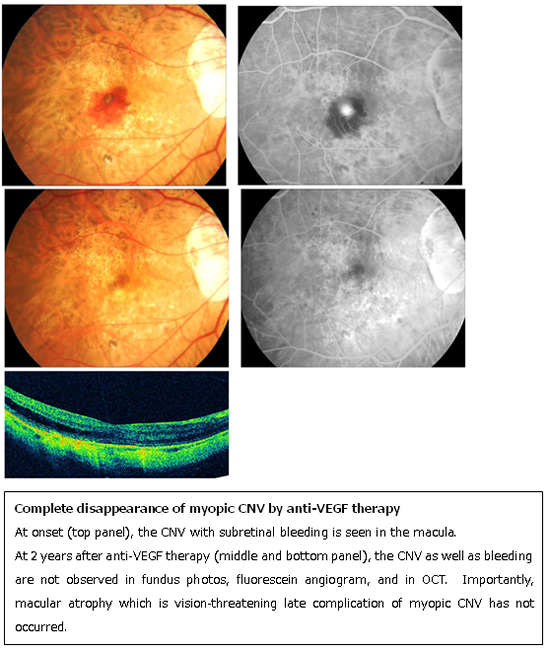
Myopic CNV tends to be smaller and less active than CNV due to age-related macular degeneration. Thus in general, myopic CNV responds very well to anti-VEGF therapies. Especially, non-subfoveal CNV sometimes disappears by anti-VEGF therapies and does not develop its late complications; macular atrophy around the regressed CNV. In that case, the patients can gain complete cure anatomically and functionally.
Novel surgical technique against MTM
Myopic CNV tends to be smaller and less active than CNV due to age-related macular degeneration. Thus in general, myopic CNV responds very well to anti-VEGF therapies. Especially, non-subfoveal CNV sometimes disappears by anti-VEGF therapies and does not develop its late complications; macular atrophy around the regressed CNV. In that case, the patients can gain complete cure anatomically and functionally.
Our future goals
Although treatment options have increased remarkably, we still have some issues that need to be solved in the future.
For example, the new treatments to prevent the development of macular atrophy around regressed CNV are expected to achieve long-term visual recovery in eyes with subfoveal CNV.
Preventive therapies against MTM are also expected.
Since no active treatments are available for myopic chorioretinal atrophy or myopic optic neuropathy, it is necessary to clarify the pathogenesis of these lesions and to establish treatments based on their pathogenesis.
However, the fundamental cause of developing myopic fundus lesions is no doubt an increase of axial length and posterior staphyloma formation.
One of our important goals is to establish new treatments to prevent axial length increase and staphyloma formation. To achieve this, we have extensively been doing basic research using animal models of myopia and are examining the effect of therapies to prevent axial length increase.
How to access our High Myopia Clinic?
If you would like to visit our High Myopia Clinic, please send an email to the following address. We will reply to you within several days.
※Note; this e-mail is only for the patients who would like to visit our High Myopia Clinic.
Contact
Publications (selected ones only)
- Morgan I, Ohno-Matsui K, Saw SM. Myopia. Lancet 379:1739-1748, 2012
- Ohno-Matsui K. Advances in Diagnosis and Treatment of Pathologic Myopia. RETINA Today 5:45-49, 2011
- Neelam K, Cheung CMG, Ohno-Matsui K, Lai TYY, Wong TY. Choroidal Neovascularization in Pathological Myopia. Progress in Retinal and Eye Research (in press)
- Ohno-Matsui K, Yoshida T. Myopic choroidal neovascularization. Natural course and treatments. Curr Opin Ophthalmol 15:197-202, 2004
- Moriyama M, Ohno-Matsui K, Modegi T, Kondo J, Takahashi Y, Tomita M, Tokoro T, Morita I. Quatitative analyses of high-resolution 3D MR images of highly myopic eyes to determine their shapes. Invest Ophthalmol Vis Sci (in press)
- Saka N, Moriyama M, Shimada N, Nagaoka N, Fukuda K, Hayashi K, Yoshida T, Tokoro T, Ohno-Matsui K. Changes of axial length measured by IOL master during two years in eyes of adults with pathologic myopia. Graefes Arch Clin Exp Ophthalmol (in press).
- Fan Q, Barathi VA, Cheng CY, Zhou X, Meguro A, Nakata I, Khor CC, Goh LK, Li YJ, Lim W, Ho CEH, Hawthorne F, Zheng Y, Chua D, Inoko H, Yamashiro K, Ohno-Matsui K, Matsuo K, Matsuda F, Vithana E, Seielstad1 M, Mizuki N, Beuerman RW, Tai1 ES, Yoshimura N, Aung T,Young TL, Wong TY, Teo YY, Saw SM. Genetic variants on chromosome 1q41 influence ocular axial 1 length and high myopia. PLOS Genetics (in press)
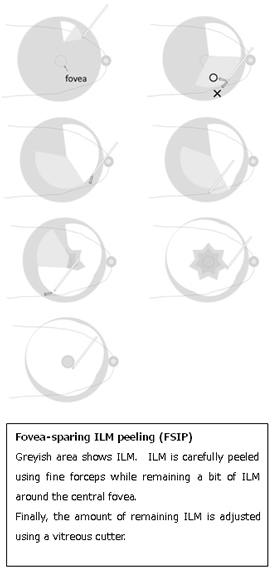
- Shimada N, Sugamoto Y, Ogawa M, Takase H, Ohno-Matsui K. Fovea-sparing internal limiting membrane peeling for myopic traction maculopathy. Am J Ophthalmol (in press)
- Asakuma T, Yasuda M, Ohno-Matsui K, Ishibashi T. Prevalence and risk factors for myopic retinopathy in a Japanese population: the Hisayama Study. Ophthalmology (in press)
- Spaide RF, Akiba M, Ohno-Matsui K. Evaluation of peripapillary intrachoroidal cavitation with sept source and enhanced depth imaging optical coherence tomography. RETINA 2012;32:1037-1044
- Akagi-Kurashige Y, Kumagai K, Yamashiro K, Nakanishi H, Nakata I, Miyake M, Tsujikawa A, Moriyama M, Ohno-Matsui K, Mochizuki M, Yamada R, Matsuda F, Yoshimura N. Vascular endothelial growth factor gene polymorphisms and choroidal neovascularization in highly myopic eyes. Invest Ophthalmol Vis Sci 2012;53:2349-2353
- Ohno-Matsui K, Akiba M, Moriyama M, Ishibashi T, Hirakata A, Tokoro T. Intrachoroidal cavitation in macular area of eyes with pathologic myopia. Am J Ophthalmol (in press)
- Ohno-Matsui K, Akiba M, Moriyama M, Shimada N, Ishibashi T, Tokoro T, Spaide RF. Acquired Optic Nerve and Peripapillary Pits in Pathologic Myopia. Ophthalmology (in press)
- Hayashi K, Shimada N, Moriyama M, Hayashi W, Tokoro T, Ohno-Matsui K. Two Year Outcomes of Intravitreal Bevacizumab for Choroidal Neovascularization in Japanese Patients with Pathological Myopia. RETINA 2012;32:687-695
- Ohno-Matsui K, Akiba M, Moriyama M, Ishibashi T, Tokoro T, Spaide RF. Imaging Retrobulbar Subarachnoid Space around Optic Nerve by Swept Source Optical Coherence Tomography in Eyes with Pathologic Myopia. Invest Ophthalmol Vis Sci 2011;52:9644-9650
- Nagaoka N, Shimada N, Hayashi W, Hayashi K, Moriyama M, Yoshida T, Tokoro T, Ohno-Matsui K. Characteristics of periconus choroidal neovascularization in pathological myopia. Am J Ophthalmol 152(3):420-427, 2011
- Ohno-Matsui K, Shimada N, Yasuzumi Y, Hayashi K, Yoshida T, Kojima A, Moriyama M, Tokoro T. Long-term development of significant visual field defects in highly myopic eyes. Am J Ophthalmol 152(2):256-265, 2011
- Tanaka Y, Shimada Y, Moriyama M, Hayashi K, Yoshida T, Tokoro T, Ohno-Matsui K. Natural history of lamellar macular holes in highly myopic eyes. Am J Ophthalmol 152(1):96-99, 2011
- Moriyama M, Ohno-Matsui K, Hayashi K, Shimada N, Yoshida T, Tokoro T, Morita I. Topographical analyses of shape of eyes with pathologic myopia by high-resolution three dimensional magnetic resonance imaging. Ophthalmology 118(8):1626-1637, 2011
- Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Hayashi W, Wang J, Yoshida T, Tokoro T, Mochizuki M. Long-term Results of Photodynamic Therapy for Choroidal Neovascularization in Japanese Patients with Pathological Myopia. Am J Ophthalmol 2011:151:137-147.
- Moriyama M, Ohno-Matsui K, Shimada N, Hayashi K, Kojima A, Yoshida T, Tokoro T, Mochizuki M. Correlation between visual prognosis and fundus autofluorescence and optical coherence tomographic findings in highly myopic eyes with submacular hemorrhage and without choroidal neovascularization. RETINA 2011:31:74-80.
- Saka N, Ohno-Matsui K, Shimada N, Sueyoshi S, Nagaoka N, Hayashi W, Hayashi K, Moriyama M, Kojima A, Yasuzumi K, Yoshida T, Tokoro T, Mochizuki M. Long-term changes of axial length in eyes of adults with pathologic myopia. Am J Ophthalmol 150:562-568, 2010
- Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, Yasuzumi K, Nagaoka N, Saka N, Yoshida T, Tokoro T, Mochizuki M. Long-term Pattern of Progression of Myopic Maculopathy: a Natural History Study. Ophthalmology 117:1595-1611, 2010
- Tanaka Y,Shimada N, Ohno-Matsui K, Hayashi W, Hayashi K, Moriyama M, Yoshida T, Tokoro T, Mochizuki M. Retro-mode Retinal Imaging of Macular Retinoschisis in Highly Myopic Eyes. Am J Ophthalmol 149:635-640, 2010
- Shimada N, Ohno-Matsui K, Yoshida T, Mochizuki M. Presence of paravascular lamellar holes in patients with idiopathic premacular fibrosis. Br J Ophthalmol 94:263-266, 2010
- Hayashi K, Ohno-Matsui K, Teramukai S, Shimada N, Moriyama M, Hayashi W, Yoshida T, Tokoro T, Mochizuki M. Comparison of visual outcome and regression pattern of myopic choroidal neovascularization after intravitreal bevacizumab or after photodynamic therapy. Am J Ophthalmol 148:396-408, 2009
- Hsiang HW, Ohno-Matsui K, Shimada N, Hayashi K, Moriyama M, Yoshida T, Tokoro T, Mochizuki M. Clinical Characteristics of Posterior Staphyloma. Am J Ophthalmol 146:102-110, 2008
- Shimada N, Ohno-Matsui K, Nishimuta A, Moriyama M, Yoshida T, Tokoro T, Mochizuki M. Detection of Paravascular Lamellar Holes and Other Paravascular Abnormalities by Optical Coherence Tomography in Eyes with High Myopia. Ophthalmolgy 115:708-717, 2008
- Shimada N, Ohno-Matsui K, Yoshida T, Sugamoto Y, Tokoro T, Mochizuki M. Progression from Macular Retinoschisis to Retinal Detachment in Highly Myopic Eyes is Associated with Outer Lamellar Hole Formation. Br J Ophthalmol 92:762-764, 2008
- Hayashi K, Ohno-Matsui K, Teramukai S, Shimada N, Moriyama M, Hara W, Tokoro T, Mochizuki M. Photodynamic Therapy with Verteporfin for Choroidal Neovascularization of Pathologic Myopia in Japanese Patients: Comparison to Nontreated Controls. Am J Ophthalmol 145:518-526, 2008
- Shimada N, Ohno-Matsui K, Yoshida T, Futagami S, Tokoro T, Mochizuki M. Development of macular hole and macular retinoschosis in eyes with myopic choroidal neovascularization. Am J Ophthalmol 145:155-161, 2008
- Shimada N, Ohno-Matsui K, Nishimuta A, Tokoro T, Mochizuki M. Peripapillary changes detected by optical coherence tomography in eyes with high myopia. Ophthalmolgy 114:2070-2076, 2007
- Moriyama M, Ohno-Matsui K, Futagami S, Yoshida T, Hayashi K, Shimada N, Kojima A, Tokoro T, Mochizuki M. Morphology and Long-term Changes of Choroidal Vascular Structure in Highly Myopic Eyes with and without Posterior Staphyloma. Ophthalmolgy114:1755-1762, 2007
- Shimada N, Ohno-Matsui K, Baba T, Futagami S, Tokoro T, Mochizuki M. Natural Course of Macular Retinoschisis in Highly Myopic Eyes without Macular Hole or Retinal Detachment. Am J Ophthalmol 142:497-500, 2006
- Shimada N, Ohno-Matsui K, Yoshida T, Yasuzumi K, Kojima A, Kobayashi K, Futagami S, Tokoro T, Mochizuki M. Characteristics of peripapillary detachment in pathologic myopia. Arch Ophthalmol 124:46-52, 2006
- Yasuzumi K, Ohno-Matsui K, Yoshida T, Kojima A, Shimada N, Futagami S, Tokoro T, Mochizuki M. Peripapillary crescent enlargement in highly myopic eyes evaluated by fluorescein and indocyanine green angiography. Br J Ophthalmol. 87: 1088-1090, 2003
- Yoshida T, Ohno-Matsui K, Yasuzumi K, Kojima A, Shimada N, Futagami S, Tokoro T, Mochizuki M . Myopic Choroidal Neovascularization: A 10-year Follow-up. Ophthalmology 110: 1297-1305, 2003
- Ohno-Matsui K, Yoshida T, Futagami S, Yasuzumi K, Shimada N, Kojima A, Tokoro T, Mochizuki M. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularization in pathologic myopia. Br J Ophthalmol 87: 570-573, 2003
- Baba T, Ohno-Matsui K, Yoshida T, Yasuzumi K, Futagami S, Tokoro T, Mochizuki M. Prevalence and Characteristics of Foveal Retinal Detachment without Macular Hole in High Myopia. Am J Ophthalmol 135:338-342, 2003
- Yoshida T, Ohno-Matsui K, Ohtake Y, Takashima T, Futagami S, Baba T, Yasuzumi K, Tokoro T, Mochizuki M. Long-term visual prognosis of choroidal neovascularizarion in high myopia. A comparison between age groups. Ophthalmology 109: 712-719, 2002
- Ohno-Matsui K, Futagami S, Yamashita S, Tokoro T Zinn-Haller arterial ring observed by ICG angiography in high myopia. Br J Ophthalmol 82: 1357-1362, 1998
- Ohno-Matsui K, Morishima N, Ito M, Yamashita S, Futagami S, Tokoro T, Nakagawa T. Indocyanine green angiography of retrobulbar vascular structures in severe myopia. Am J Ophthalmol 123: 494-505, 1997
- Ohno-Matsui K, Morishima N, Ito M, Tokoro T. Posterior routes of choroidal blood outflow in high myopia. Retina 16: 419-425, 1996
- Ohno-Matsui K, Morishima N, Ito M, Yamashita S, Tokoro T. Subretinal bleeding without choroidal neovascularization in pathologic myopia. Retina 16: 196-202, 1996
- Ohno-Matsui K, Tokoro T. The progression of lacquer cracks in pathologic myopia. Retina 16: 29-37

